Technical Review: Optimizing mRNA delivery: A microfluidic exploration of DOTMA vs. DOTAP lipid nanoparticles for GFP expression on human PBMCs and THP-1 cell line
- aprilt97
- Mar 19
- 4 min read
Updated: 5 days ago
Authors: Erwin Pavel Lamparelli, Elena Ciaglia, Maria Camilla Ciardulli, Valentina Lopardo, Francesco Montella, Alessandro Annibale Puca, Giovanna Della Porta
Affiliation: University of Salerno, Via S. Allende, Baronissi, SA 84081, Italy
Background
This paper contributes to the rapidly expanding field of nucleic acid delivery systems, specifically focusing on lipid nanoparticle (LNP) formulations for mRNA delivery. The research sits at the intersection of pharmaceutical sciences, biomedical engineering, and molecular biology, with significant implications for vaccine development and gene therapy applications. Since the successful deployment of mRNA vaccines for COVID-19, optimizing LNP formulations has become a critical research priority to enhance transfection efficiency, reduce cytotoxicity, and improve manufacturability.
Cationic lipids play a crucial role in LNP formulations by facilitating the encapsulation of negatively charged mRNA through electrostatic interactions. Within this context, DOTMA (1,2-di-O-octadecenyl-3-trimethylammonium propane) and DOTAP (1,2-dioleoyl-3-trimethylammonium-propane) represent two of the most widely used cationic lipids in nucleic acid delivery systems, yet comprehensive comparative studies evaluating their relative performance in microfluidic-generated LNPs have been limited. The study capitalizes on recent advancements in microfluidic technologies that allow for precise control over mixing parameters and reproducible nanoparticle production, addressing a significant need for standardized comparison of these lipid carriers.
Materials and Methodology
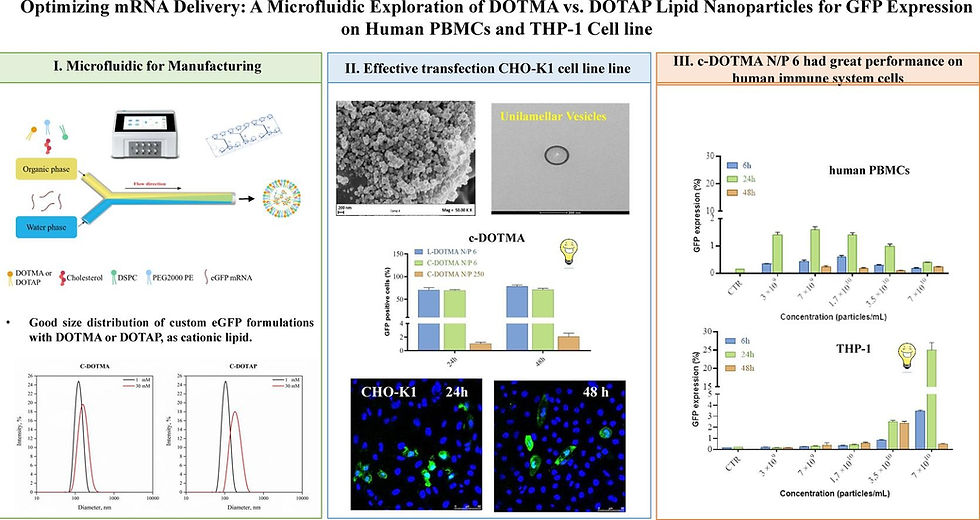
The researchers employed a microfluidic approach to systematically compare LNP formulations containing either DOTMA or DOTAP as the cationic lipid component. The study utilized the Flex-M instrument and LipidFlex reagent from PreciGenome for consistent lipid nanoparticle preparation, allowing for controlled nanoprecipitation and reproducible particle size distribution. This methodological choice aligns with current trends in LNP production, as microfluidic mixing enables more precise control over critical formulation parameters compared to conventional bulk mixing techniques.
The research team developed multiple LNP formulations incorporating either DOTMA or DOTAP with helper lipids and evaluated them against commercial formulations. The nanoparticles were characterized for physical properties including size, polydispersity index (PDI), zeta potential, and mRNA encapsulation efficiency. For functional assessment, the LNPs were loaded with mRNA encoding enhanced Green Fluorescent Protein (eGFP), and transfection efficiency was evaluated in human peripheral blood mononuclear cells (PBMCs) and THP-1 monocytic cell line. These cell types were strategically selected for their relevance to immune responses and vaccine applications.
The experimental design included optimization of critical parameters such as the nitrogen/phosphate (N/P) ratio, lipid composition, and flow rate ratios within the microfluidic system. The study appears to have carefully controlled for variables that might influence transfection outcomes, enabling a fair comparison between the DOTMA and DOTAP formulations.
Results and Key Findings
The paper presents a detailed comparison of DOTMA and DOTAP-based LNPs, revealing distinct differences in their physicochemical properties and biological performance. The microfluidic preparation method yielded uniform nanoparticles with narrow size distributions for both lipid types, confirming the advantages of this production approach for generating consistent LNP formulations.
DOTMA-containing LNPs demonstrated different encapsulation efficiencies and release kinetics compared to DOTAP formulations, which appeared to correlate with variations in cellular uptake and subsequent eGFP expression. The study provides valuable insights into how the structural differences between these two cationic lipids influence their interaction with helper lipids, mRNA cargo, and ultimately their transfection capabilities in human immune cells.
The researchers observed cell type-specific responses, with different optimal formulations identified for PBMCs versus THP-1 cells. This finding highlights the importance of tailoring LNP composition to specific target cells when designing delivery systems for clinical applications. The study also examined the influence of the lipid/mRNA ratio on transfection outcomes, identifying optimal parameters that maximize protein expression while minimizing cytotoxicity.
The benchmarking against commercial formulations provided context for evaluating the performance of the custom-designed LNPs, demonstrating how systematic optimization of formulation parameters can yield improvements in transfection efficiency.
Evaluation and Contribution
The study's primary strength lies in its systematic approach to comparing two widely used cationic lipids using standardized microfluidic production methods. By controlling for production parameters and evaluating performance across multiple cell types, the research provides practical insights for rational LNP design. The use of microfluidic technology represents a methodological advantage, as it enables precise control over mixing conditions that influence nanoparticle characteristics and reproducibility.
The authors appropriately evaluated transfection efficiency using GFP expression as a quantifiable readout, which allows for direct visualization and measurement of successful mRNA delivery and translation. This approach provides clear evidence for the comparative performance of the different formulations.
From an experimental design perspective, the research demonstrates rigor in its comprehensive characterization of the LNP formulations and their biological effects. The inclusion of both primary human cells (PBMCs) and a cell line (THP-1) strengthens the translational relevance of the findings.
One potential limitation might be the specific nature of the mRNA payload (eGFP), which may not fully predict the behavior of therapeutic mRNAs with different sequence characteristics or stability profiles. Additionally, while in vitro studies provide valuable insights, they cannot fully predict in vivo performance where additional biological barriers exist.
The study makes a significant contribution to the field by providing evidence-based guidance for selecting between DOTMA and DOTAP in microfluidic-produced LNPs for specific cell targets. This information is particularly valuable for researchers developing mRNA therapeutics and vaccines, where optimizing delivery efficiency remains a critical challenge.
Conclusion
This paper represents a valuable addition to the growing body of literature on mRNA delivery systems. By directly comparing DOTMA and DOTAP in carefully controlled microfluidic-generated LNPs, the authors provide practical insights that can guide formulation decisions for different application scenarios. The systematic approach to optimization and characterization sets a methodological standard for comparative studies of lipid components in nucleic acid delivery systems.
The findings reinforce the importance of tailoring LNP formulations to specific cell types and applications, highlighting that optimal delivery systems may require customization rather than one-size-fits-all approaches. As the field of mRNA therapeutics continues to expand, such comparative studies will be increasingly important for rational carrier design and development.
For more details on this innovative research, you can access the full paper at:
Comments