
Introduction
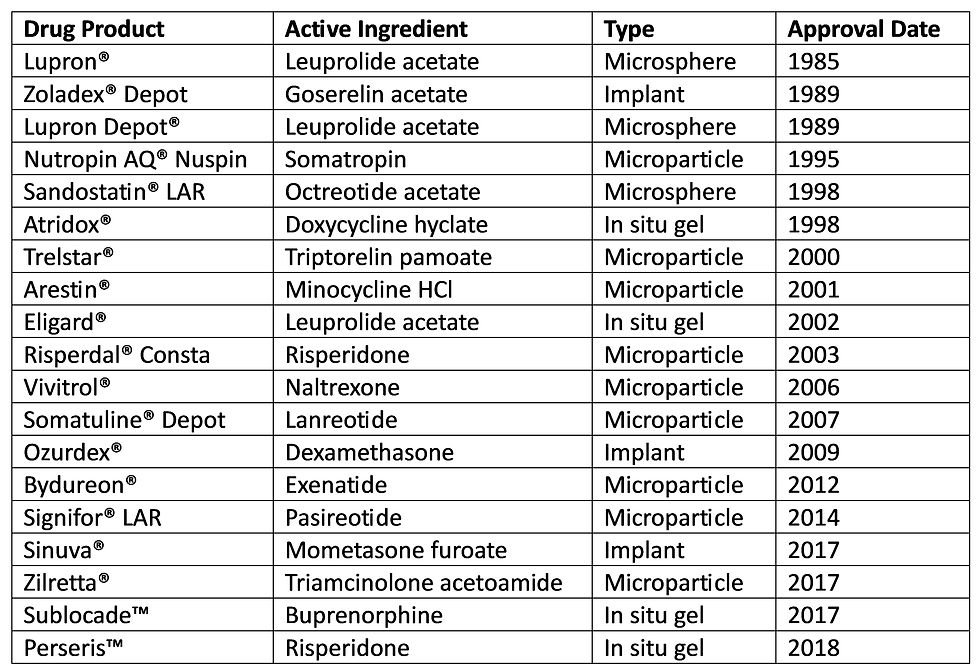
Poly(lactic co-glycolic acid) (PLGA) is a synthetic biodegradable polymer commonly used in drug delivery applications requiring sustained release. It was FDA approved for 19 drug formulations as of 2019 thanks to its high biocompatibility and biodegradability [1], and it may be applied in various forms such as tissue scaffolds, grafting materials, nanoparticles, or microparticles. The majority of these FDA approved formulations are delivered as PLGA microparticles, which consist of a solid PLGA core carrying a drug.
Drug Release Mechanisms
One major benefit of PLGA microparticles as a drug delivery platform is their ability to provide controlled drug release. This is a complex phenomenon with several stages and interdependent mechanisms, which include drug diffusion, swelling, and hydrolysis [2].
Diffusion occurs when a water soluble drug permeates through the pores in the PLGA network. These pores may either be already present during microparticle formation, or opened from continuous interactions between the microparticle and water.
Swelling involves the hydration of PLGA chains and an increase in microparticle volume. As the polymer chains become more mobile, drug diffusion becomes easier.
Hydrolysis degrades the PLGA into shorter chains, reducing its molecular weight and releasing any drugs embedded in the polymer network. This eventually ends with the total degradation of the PLGA microparticle.
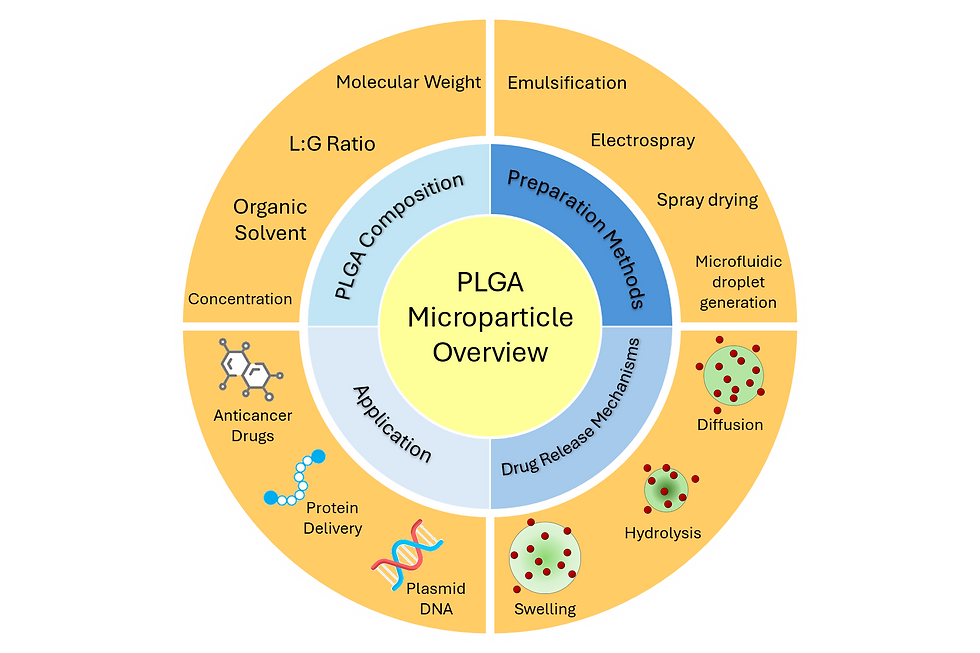
Fig 1. Overview of relevant parameters in PLGA microparticle drug delivery
Tuning PLGA's Properties
Several parameters may be adjusted to tune PLGA's degradation rate, which in turn affects its rate of drug release. These include molecular weight, lactide:glycolide (L:G) ratio, or the organic solvent used to dissolve it. Depending on how PLGA is being applied, additional factors must be considered for successful drug release. For microparticles in particular, the chief concern is size distribution [3], which affects the uniformity of drug loading and therefore degradation rate.
Traditional Microparticle Preparation
PLGA microparticles are traditionally prepared in a variety of ways, such as emulsion-solvent evaporation, electrospray, and spray drying.
Emulsion-solvent evaporation (emulsification) involves dissolving PLGA in an organic solvent and adding this to an aqueous phase containing surfactant. This forms PLGA microparticles through emulsion, and the microparticles remain once the solvent has evaporated. The process may also be repeated with the resulting microparticles for a second emulsion. Emulsification is the most common approach for PLGA microparticle synthesis due to its ease of use and limited control over particle size. However, it is limited by poor particle size uniformity and difficulty with scaling up production.
Electrospray (electrospinning) uses a syringe pump to inject PLGA solution through a nozzle When a high voltage is applied between the nozzle and a collection substrate, the PLGA solution forms a Taylor cone of droplets, which in turn become microparticles on the collection substrate. Electrospinning offers several parameters for size tuning of microparticles, though it still faces challenges with scaling up production.
Spray drying atomizes the PLGA solution through a nozzle into heated air. The droplets formed at the nozzle are dried upon contact with the air to form solid microparticles. Spray drying is well suited for automation since it does not require a separate drying step. However, particle adhesion to the spray dryer remains a major drawback.
Microfluidic Droplet Generation
Microfluidic droplet generation presents an appealing solution for monodisperse PLGA microparticle synthesis with good potential for scaled up production. By emulsifying a dispersed phase in an immiscible continuous phase, uniform micron sized droplets may be generated at high throughput, typically hundreds or even thousands per second. As droplet generation traditionally involves an oil-based and water-based phase, this emulsion is denoted as either water-in-oil or oil-in-water depending on which phase is being dispersed.
For PLGA microparticles, PLGA is dissolved in the oil phase for an oil-in-water emulsion. The oil phase may be one of several organic solvents; dichloromethane (DCM) is traditionally the most common, though its toxicity has promoted substitution with other solvents like dimethyl carbonate (DMC) [4]. A surfactant such as poly(vinyl alcohol) (PVA) is also typically dissolved in the water phase, as this increases the resulting emulsion's stability. Microparticles will still shrink shortly after production independently of PLGA degradation. This occurs because the organic solvent will diffuse into the aqueous buffer and evaporate, leaving the microparticle as a condensed PLGA bead.
Similar to emulsification, microfluidic droplet generation allows the possibility of double emulsion. However, this is simpler with microfluidic droplet generation, since the user only needs to switch to a different microfluidic channel design. The choice between single or double emulsion largely depends on the type of drug being delivered; single emulsion is more suitable for hydrophobic drugs, while double emulsion is better for hydrophilic drugs.
Traditional Methods | Microfluidic Droplet Generation (PreciGenome) | |
Particle Size Distribution | ~20% | ~2% |
Reproducibility | Low | High |
Live Size Tuning | No | Yes |
Continuous Production | No | Yes |
Materials and Methods
The following application note will detail how to use PreciGenome's iFlow controller to produce monodisperse PLGA microparticles dissolved in DMC.
Reagents
Dispersed Phase: Poly-(lactic-co-glycolic acid) (45 kDa, 50:50 lactide:glycolide, Sigma) dissolved in dimethyl carbonate (≥ 99%, Sigma).
Continuous Phase: Poly(vinyl alcohol) (13-23 kDa, 87-89% hydrolyzed, Sigma) dissolved in deionized water (MiliQ).
Dispersed phase preparation
PLGA was dissolved to 1% (w/v) in DMC with sonication for 30 minutes before being filtered through a 0.22 µm filter. The resulting solution was further sonicated for 10 minutes immediately before use.
Continuous phase preparation
PVA was dissolved in DI water with boiling for 1 hour. DI water was added as needed after dissolving to achieve a final concentration of 1% (w/v). The resulting solution was filtered through a 0.22 µm filter and further sonicated for 10 minutes immediately before use.
Droplet generation setup
An iFlow Touch pressure controller (PG-MFC-8CH) was used to handle fluids. Micronit glass droplet generation chips (sideconnect, 10 and 50 μm nozzles) were used for microparticle synthesis. 2 µm inline filters (Cole Parmer) were used to further prevent debris buildup in the droplet generation chips. Finally, a high speed imaging system (PG-HSV-M) was used to monitor droplet production.
Microparticle collection
Microparticles were collected directly from the droplet generation chip. A BioRad ZOE fluorescent cell imager was used to observe droplets after collection, with special attention to microparticle precipitation. As DMC diffuses from a droplet into the surrounding aqueous solution, the PLGA core precipitates into a solid microparticle smaller than the original droplet.

Fig 2. Droplet generation setup schematic
Results
Fig 3. PLGA microparticles before (A) and after (B) 60 s. DMC evaporation brings the final size to 20 µm from 62 µm. No further shrinking is observed after 60 s.
Fig 4. PLGA microparticle size tuning. Increasing the dispersed phase pressure increases droplet size. The initial droplet size is more affected by this than final droplet size.

Fig 5. PLGA microparticle size tuning. Changing the nozzle size greatly affects possible droplet size. These microparticles produced from a 10 µm nozzle chip had an initial size of 6 µm, which was further reduced to 1.5 µm after shrinkage.
Conclusion
The iFlow platform is a robust and convenient tool for PLGA microparticle synthesis. By tuning the pressure during operation, users can achieve excellent control over droplet size and production rate. Combined with our high speed imaging system for easy visualization, PreciGenome thus provides an economical solution from early discovery to pre-clinical studies and scaled up production.
References
Park et. al. Injectable, long-acting PLGA formulations: Analyzing PLGA and understanding microparticle formation
Rahmani et. al. The recent insight in the release of anticancer drug loaded into PLGA microspheres
Bahl et. al. Dynamic changes in size distribution of emulsion droplets during ethyl acetate-based microencapsulation process
Kim et. al. Preparation of Monodisperse ENX-Loaded PLGA Microspheres Using a Microfluidic Flow-Focusing Device








Comments